
Breaking local symmetry—why water freezes but silica forms a glass
4.7 (502) In stock

4.7 (502) In stock
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water's cousin, silica, exhibits wayward behavior when cooled that has long puzzled scientists.
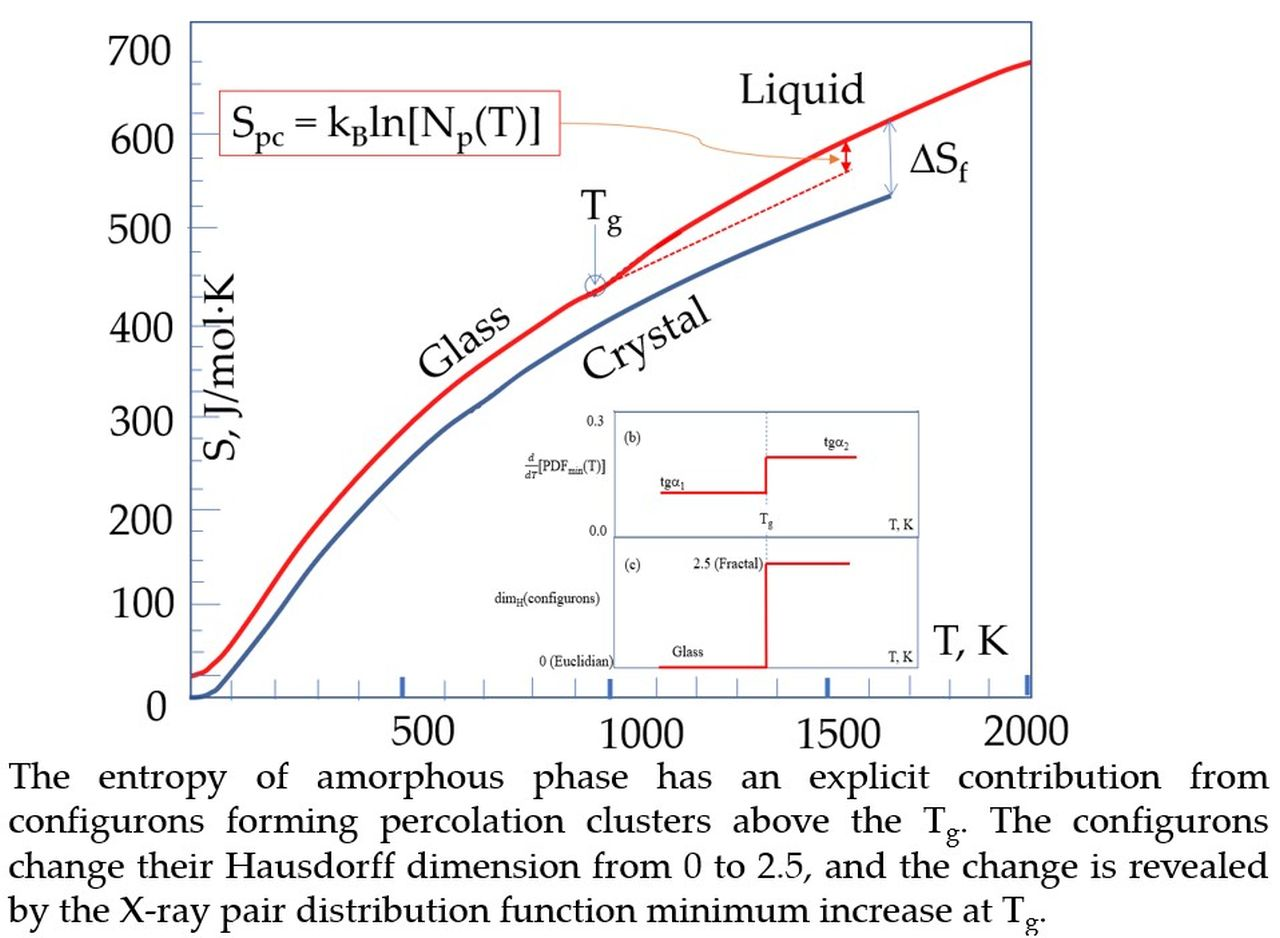
Materials, Free Full-Text

Critical cooling rate versus reduced glass transition temperature T rg

Glass transition - Wikipedia
Does water become less dense as it becomes colder or only when it reaches freezing temperature? - Quora

Models of substructures participating in the silica network and

Structural origin of the anomalous properties of SiO2 glass under pressure

Q&A: How Moving Water Freezes – SKY LIGHTS

Various types of defects artificially produced on glass surfaces. From

Structure and dynamics of nanoconfined water and aqueous solutions

Fast vs slow water—explaining the fragile-to-strong transition
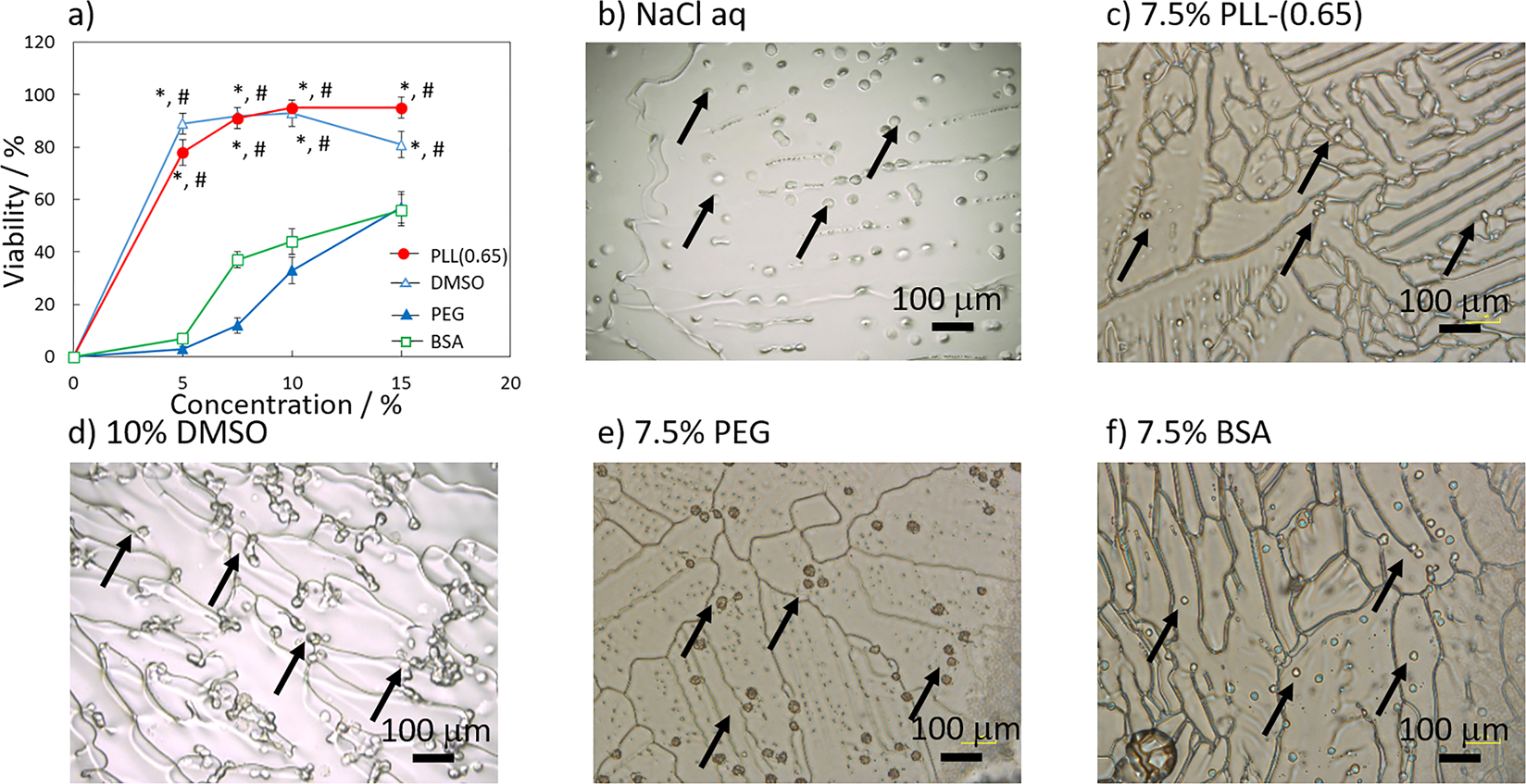
Molecular mechanisms of cell cryopreservation with polyampholytes studied by solid-state NMR

Fracture surface of silica optical fibers (the somewhat darker ring is
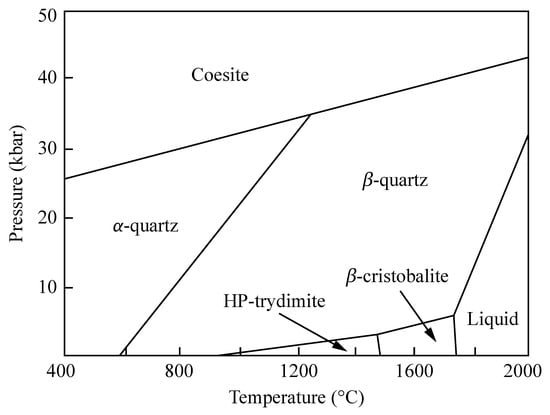
Materials, Free Full-Text

Understanding water's anomalies with locally favoured structures

Is glass transition driven by thermodynamics?