
What is the change in internal energy (in J) of a system that
4.6 (113) In stock

4.6 (113) In stock
I found an increase of 3100J Have a look
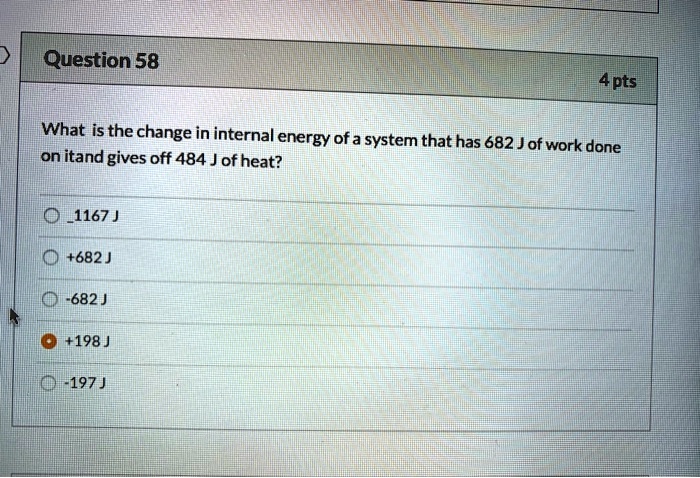
SOLVED: Question 58 #4 pts What is the change in internal energy

Calculate the change in internal energy delta E for a system that is giving off 25 0kJ of heat and i

500 J of heat was supplied to a system constant volume. It resulted in the increase of temperature of the system from 20^oC to 25^oC. What is the change in internal energy
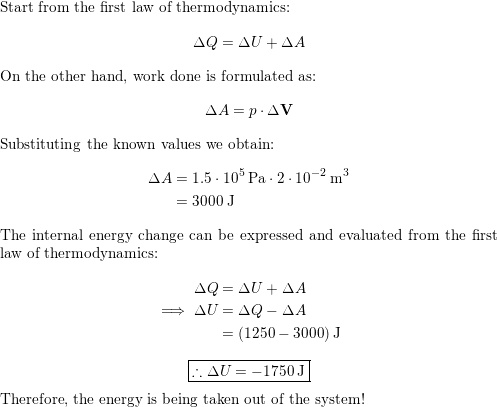
A chemical reaction transfers 1 250 J of thermal energy into

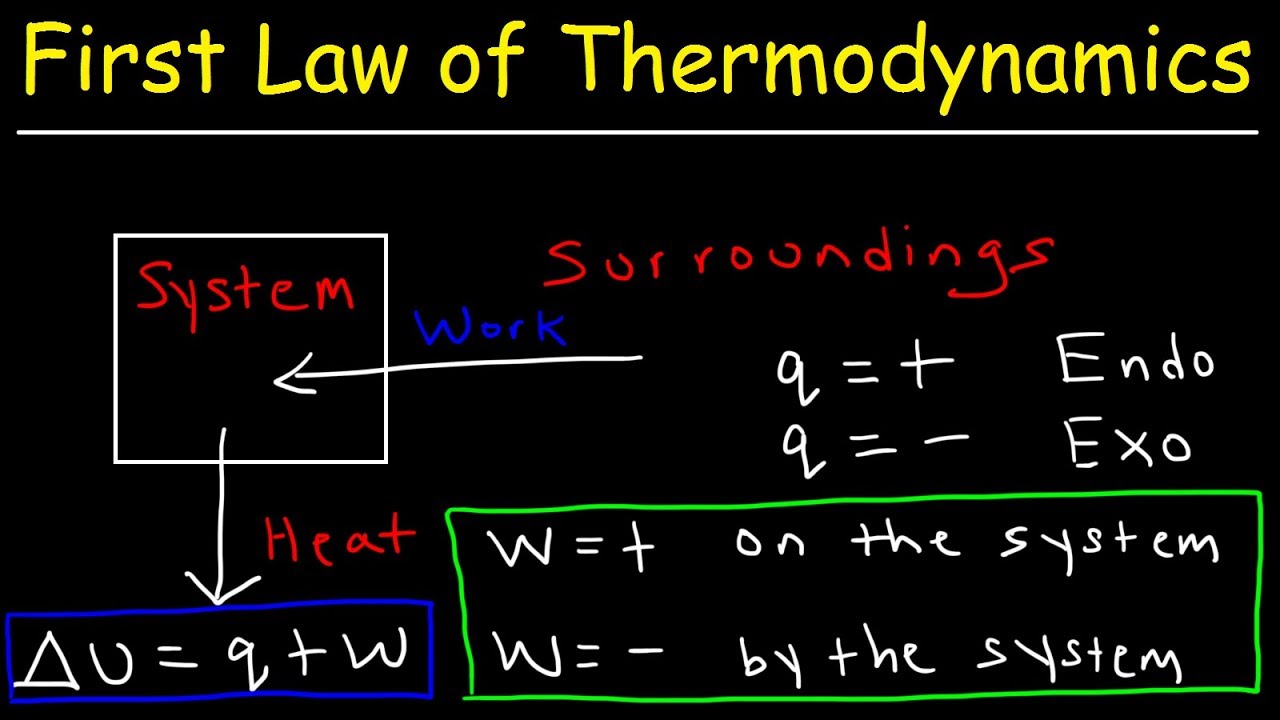
PPT - Ch12.1 – Thermal Energy PowerPoint Presentation, free download - ID:3067599
CaptionSync Smart Player™

Ch6.1 The Nature of Energy (hustle!) - ppt download
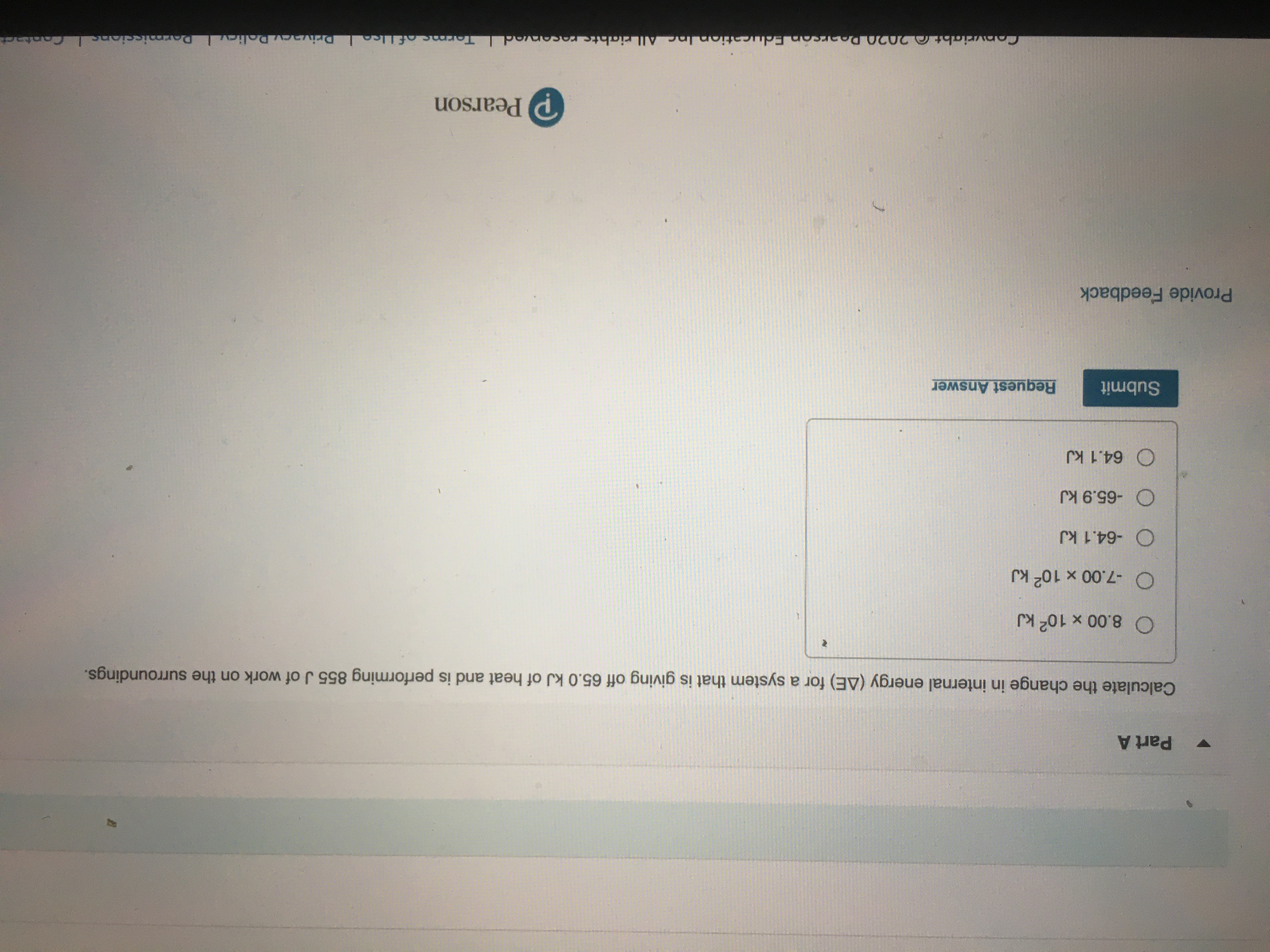
Answered: Calculate the change in internal energy…
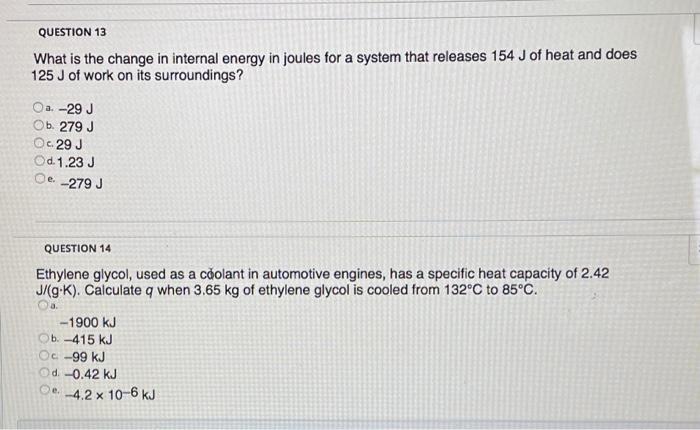
Solved QUESTION 13 What is the change in internal energy in
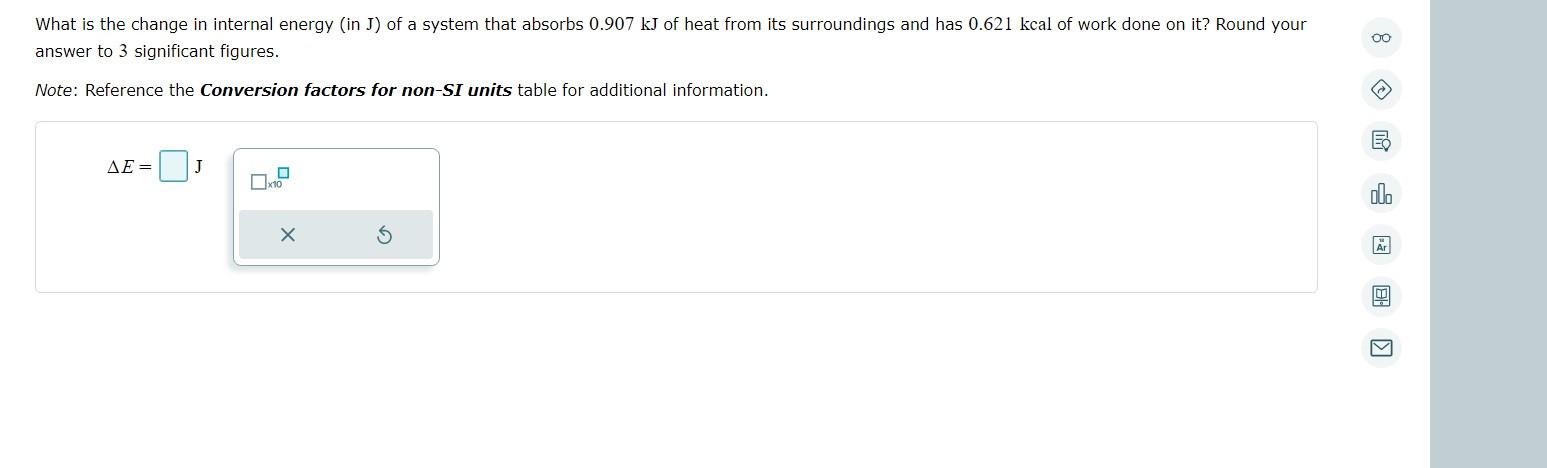
Solved What is the change in internal energy (in J) of a
Answered: What is the change in internal energy…
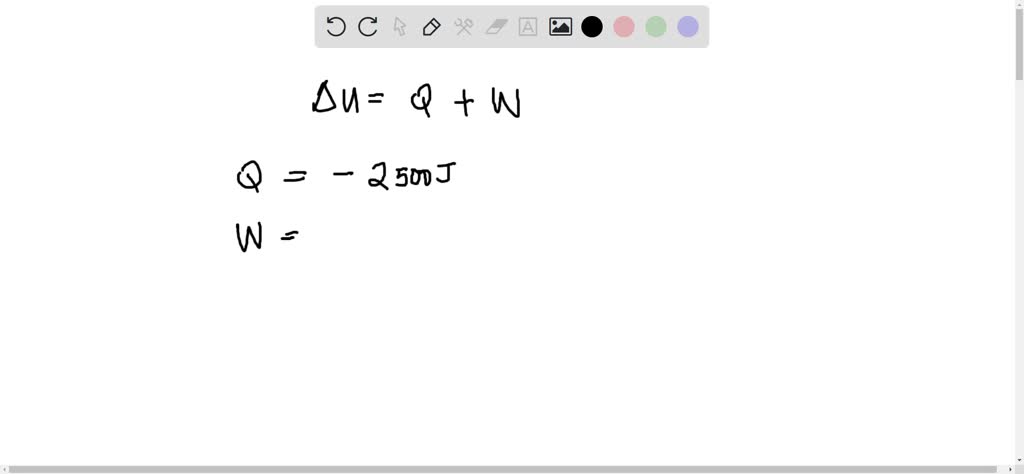
SOLVED: The change in the internal energy of a system that releases 2,500 J of heat and that does 7,655 J of work on the surroundings is J.
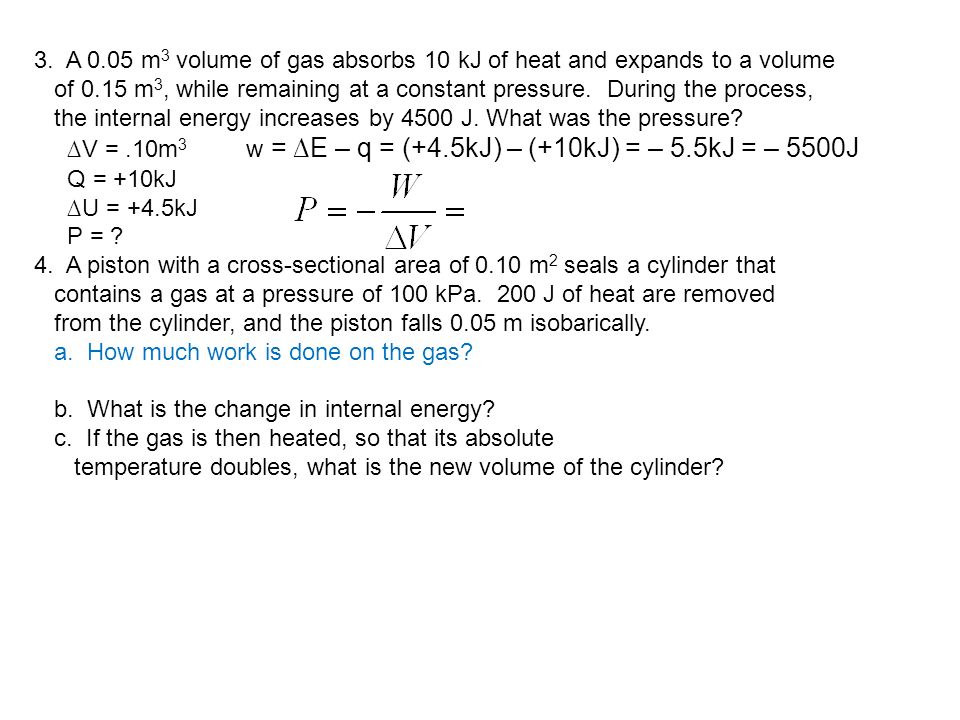
Ch6.1 The Nature of Energy (hustle!) - ppt download

Ch6.1 The Nature of Energy (hustle!) - ppt download