The vapour pressure of a solution having 2.0 g of solute X (gram
4.9 (683) In stock
4.9 (683) In stock
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8
The vapour pressure of a solution having 2.0 gram of solute X in hundred gram of CS2 is it 848.9 torr. the molecular formula of the solute is

PPT - Chapter 12—Solutions and Properties PowerPoint Presentation, free download - ID:260322
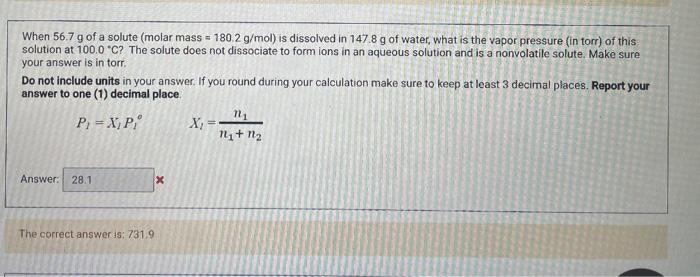
Solved When 56.7 g of a solute (molar mass =180.2 g/mol ) is

A solution containing 8.6 g L^-1 of urea is isotonic with a 5 %(w / v) solution of unknown sol
PPT - Chapter 7: Solutions and Colloids PowerPoint Presentation

Sat chemistry notes by Andora Conti - Issuu

Chapter 16 Colligative properties of solutions
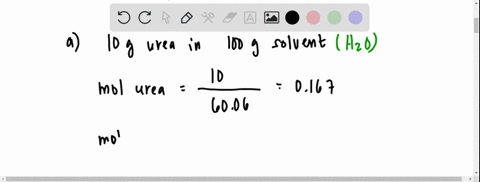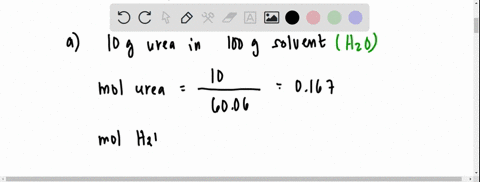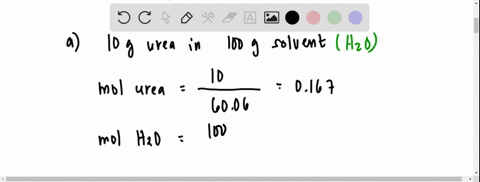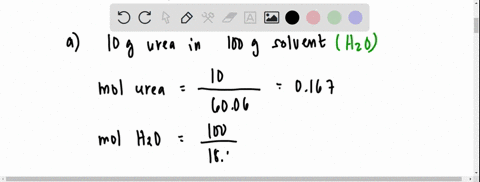
SOLVED: The vapour pressure of a solution having 2.0 g of solute X

The vapour pressure of a dilute aqueous solution of glucose is `700 mm` of `Hg` at `373 K`.

Solutions (Colligative Properties, Abnormality in Molar Mass) The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-1) in 100 g of CS, (vapour

chem 178 ch 13 Flashcards
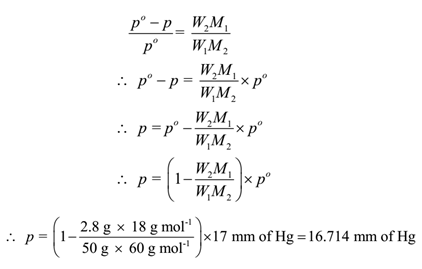
Lowering of Vapour Pressure: Numerical problems with solutions

Properties of aqueous solutions. A treatise against osmotic and activity coefficients - ScienceDirect

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..