SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through
4.7 (741) In stock

4.7 (741) In stock
VIDEO ANSWER: Hello. Here we are given a PV diagram. So it's in shape of a square. Right? And the process and volume R P I If you have N B I. Here we have A V I. And here we have N. B I. Right? And it's given that N is equal to 3.6. So an ideal
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
Marathi] One mole of an ideal gas is initially kept in a cylinder wit
Ideal gas law - Wikipedia

If one mole of an ideal gas at P1,V1,T is allowed to expand reversibly and isothermally A toB its pressure is reduced to 12 of original pressure see figure. This is followed

Solved] If one mole of an ideal gas at (P1, V1) is allowed to expand

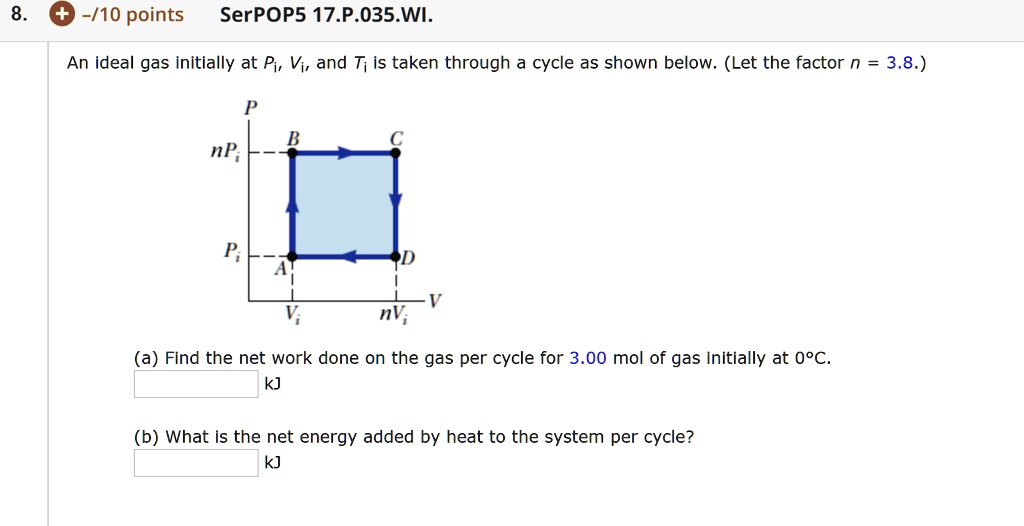
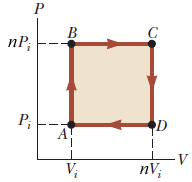
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n = 3.3.)
Solved An ideal gas initially at Pi, Vi, and Ti is taken
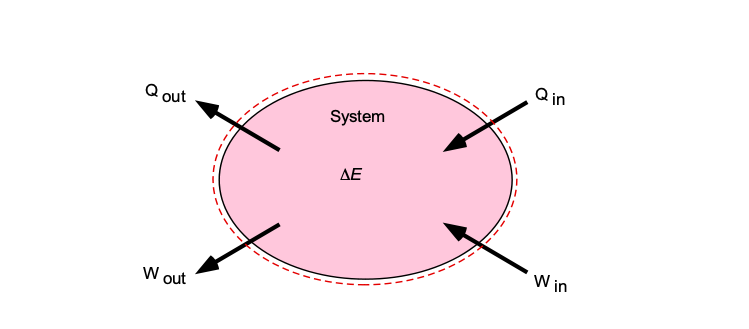
Energy Equation & Bernoulli's Equation – Introduction to Aerospace Flight Vehicles

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of

1 mole of an ideal gas undergoes reversible isothermal expansion from an initial volume V_{1} to a final volume 10V_{1} and does 10 KJ of work. The initial pressure was 1times 10^{7}PaCalculate V_{1}
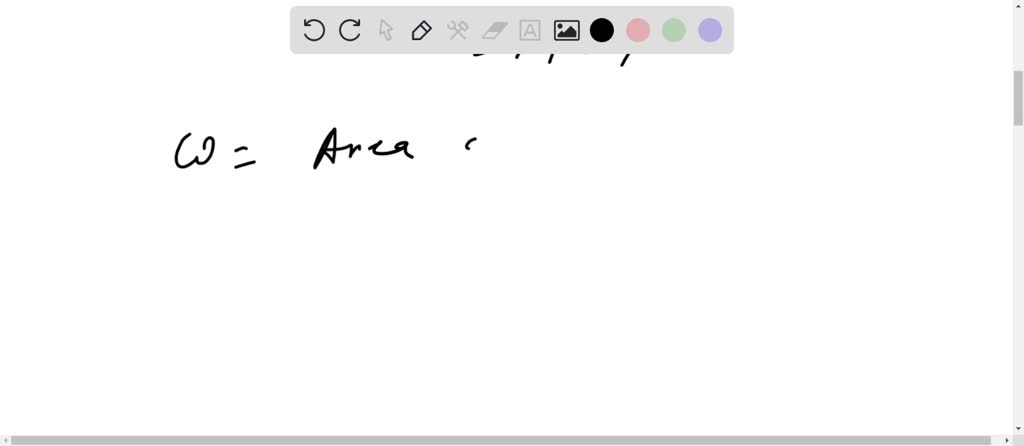
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n 3.6.) nP; P; nV; (a Find the net work done