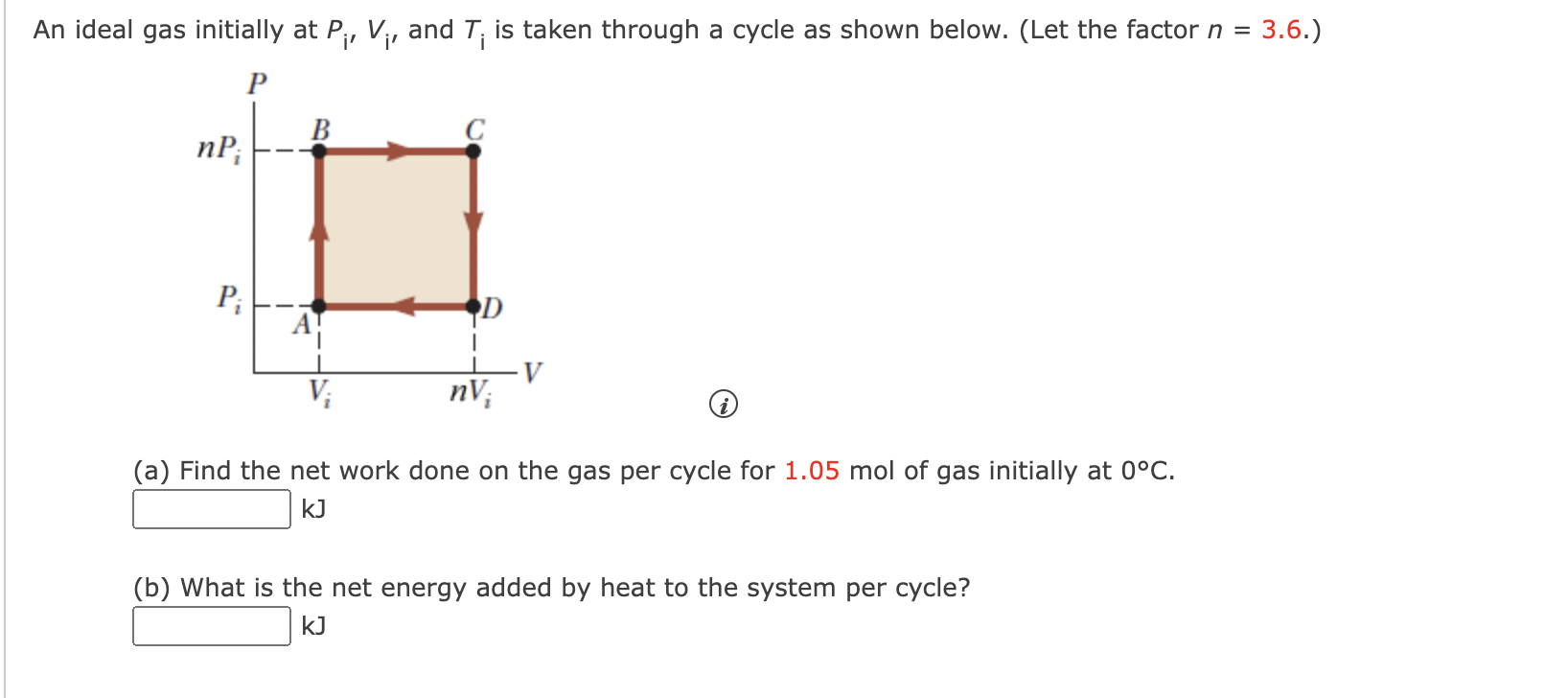
Solved An ideal gas initially at Pi, V;, and T; is taken
4.8 (351) In stock

4.8 (351) In stock

Figure 20-29 showsa reversible cycle through which 1.00 mol of a monatomic ideal gas is taken. Volume V_{c}=8.00 V_{b} . Process b c is an adiabatic expansion, with p_{b} =10.0 mathrm{atm} and

An ideal gas is taken through a process in which pressure and volume vary as `P = kV^(2)`.
Two moles of an ideal gas is compressed isothermally and reversibly from a volume 2L to 0.5L at initial pressure of 1 atm . the work done by gas i
An ideal gas goes isothermally at temperature T K from initial state (3P,..
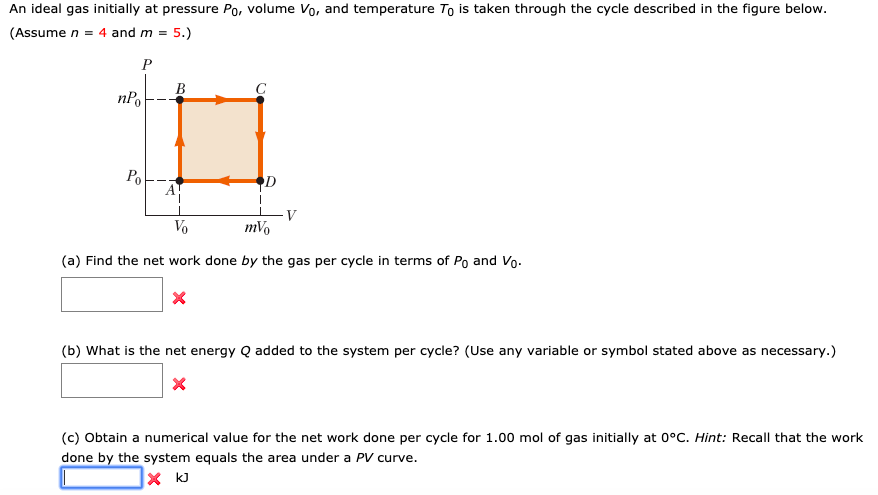
Solved An ideal gas initially at pressure P0, volume V0, and

SOLVED: initially at P, Vi and Ti is taken through cycle as shown below: (Let the factor n = 3.8.) An ideal gas Find the net work done on the gas per
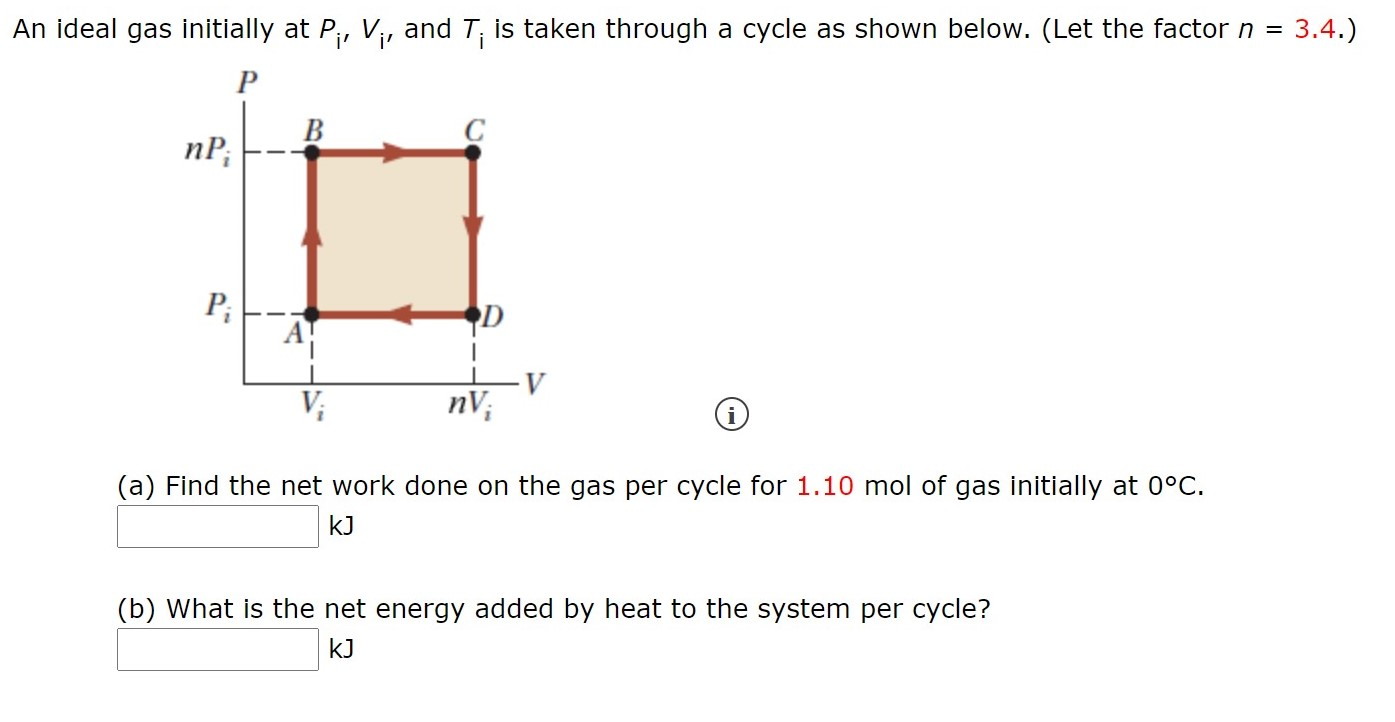
Solved An ideal gas initially at Pi, V;, and T; is taken
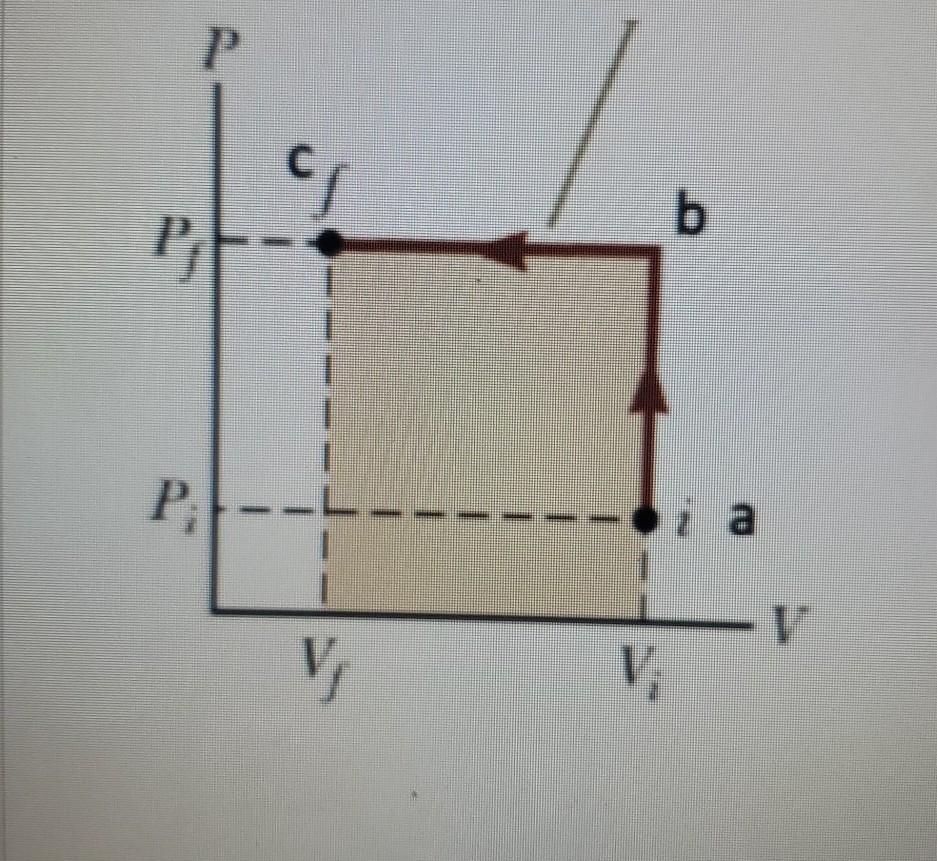
Solved An ideal gas is taken from initial to final states
1st law
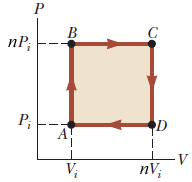
Solved An ideal gas initially at Pi, Vi, and Ti is taken

Isobaric Process - an overview
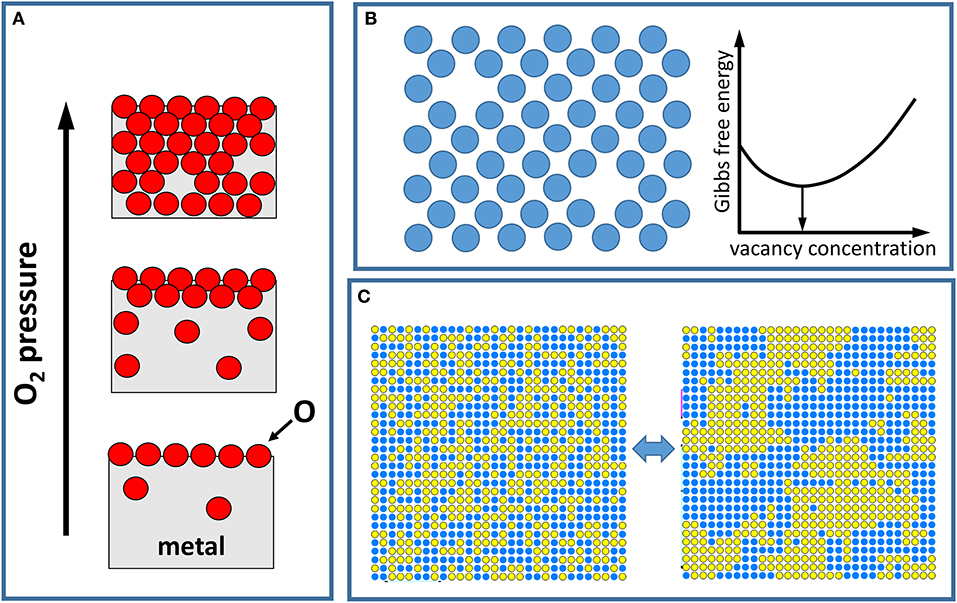
Frontiers First-Principles Atomistic Thermodynamics and Configurational Entropy
1st law
the pressure of an ideal gas varies according to law P=P_0 AV^2 where p0 and A are positive cons†an ts .find the highest temperature attained by the ga
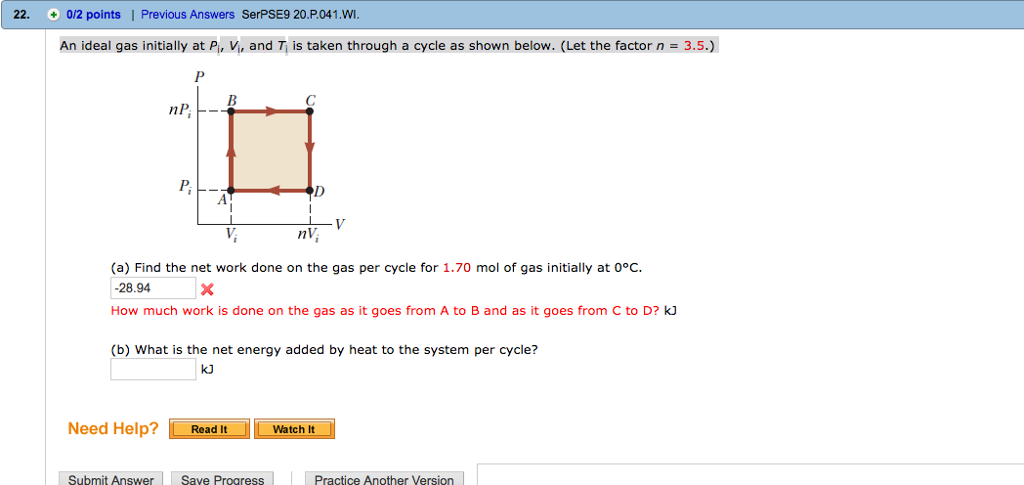
Solved An ideal gas initially at Pi, Vi, and Ti is taken