What is the value of compressibility factor in terms of vander
4.6 (174) In stock
4.6 (174) In stock
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

The role of the compressibility factor Z in describing the volumetric behavior of gases

Non-Ideal Gas Behavior Chemistry: Atoms First

If Z is a compressibility factor, van der Waals equation at low pressure ..
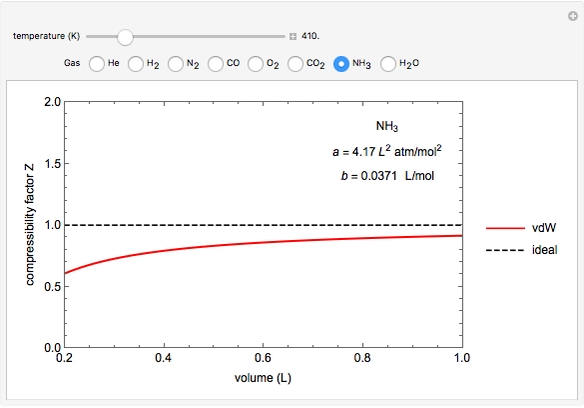
Compressibility Factors for van der Waals Gases - Wolfram Demonstrations Project
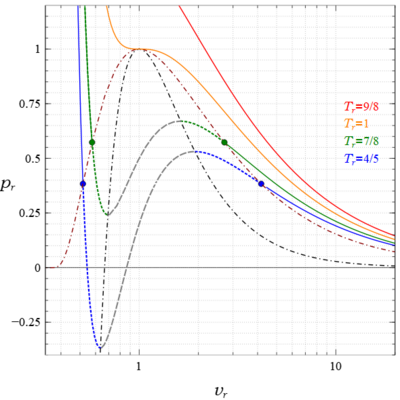
Van der Waals equation - Wikipedia

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

SOLVED: (a) State the van der Waals gas equation, defining all its terms and their units [3 MARKS] (b) Derive an expression for the excluded volume of a gas (per mole of

SOLVED: physical chemistry Using the expressions for the critical constants in terms of the van der Waals constant, calculate the value of the compressibility factor at the critical state [Answer: 3/8]

Compressibility factors of air under the specified pressure and
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect

Explain how the compression factor varies with pressure and

What is the value of z (compressibility factor) for a vander waal gas at critical
The value of compression factor at the critical state of a vander waals gas is