The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
4.9 (363) In stock
4.9 (363) In stock
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

16. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g of CS, (vapour pressure = 854 torr) is 848.9
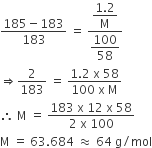
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o
5g sulphur is present in 100 g of CS(2). DeltaT(b) of solution 0.954 a

The vapour pressure of a solution having 2.0g of a solute X(molar mass 32gmol−1) in 100g of CS2 (vapour


The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..


Solved 1. Assuming Raoult's Law applies, calculate the vapor

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..

Solution.pdf - Chemistry - Notes - Teachmint
How to calculate the vapour pressure of a solution prepared by dissolving 211.6 g MgBra (184.1 g/mol) in 109 g of water - Quora
The vapor pressure of a solution having 2 gram of solute X ( gram atomic mass =32 gram/mol ) in 100 gram of CS_2 ( vapour pressure = 854 torr ) is
⏩SOLVED:Calculate the vapor pressure at 25^∘ C of a solution…