
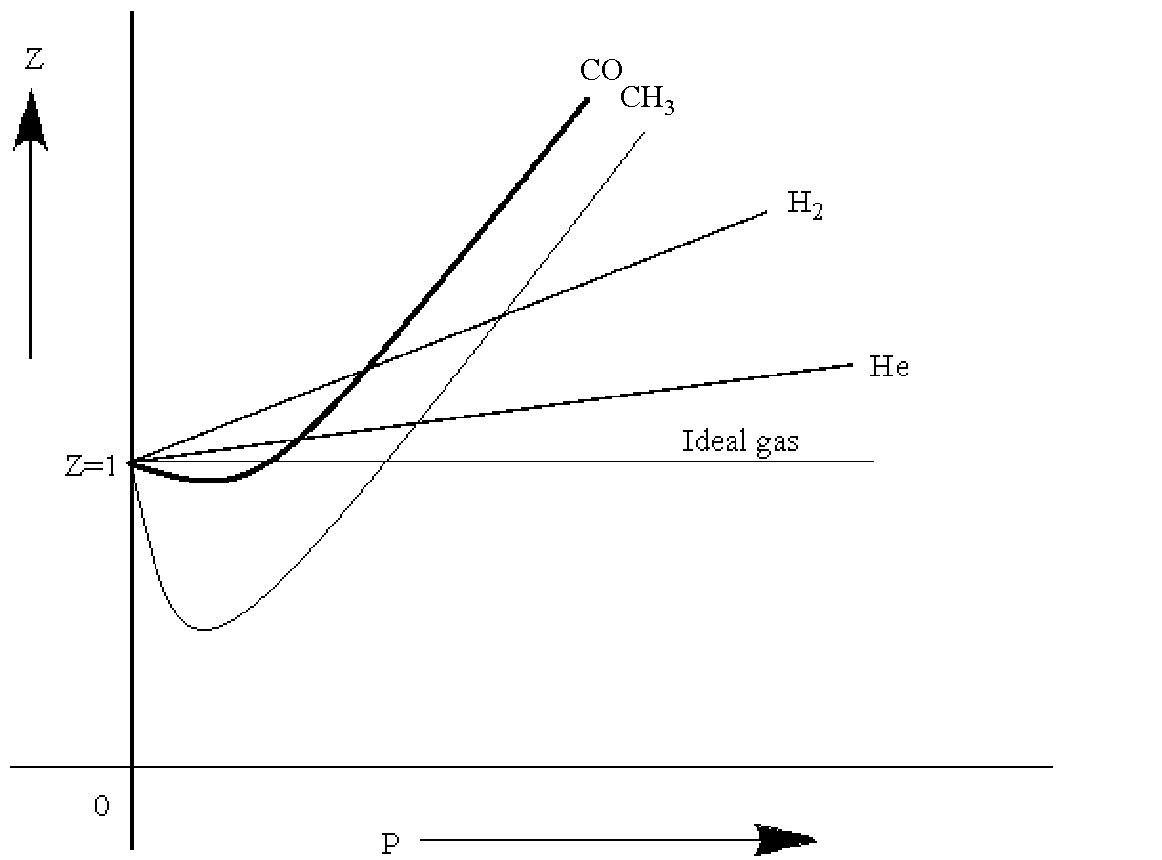
The compressibility factor Z a low-pressure range of all gases
4.7 (119) In stock

4.7 (119) In stock
Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor z at a lowpressure range of all gases except hydrogen is
Click here👆to get an answer to your question ✍️ The compressibility factor Z a low-pressure range of all gases except hydrogen is-Z-1- displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-Pb-RT-Z - - 1 - displaystylefrac-Pb-RT-
The van der Waals equation for real gases is -P-aVm2-Vm-x2212-b-RT

Determine Compressibility of Gases

The compressibility factors for 1 mole of real gases at low pressure, high pressure and that of gases of very low molar masses are Z1, Z2 and Z3. These are

Compressibility factor (z): real gases deviate from ideal behav-Turito
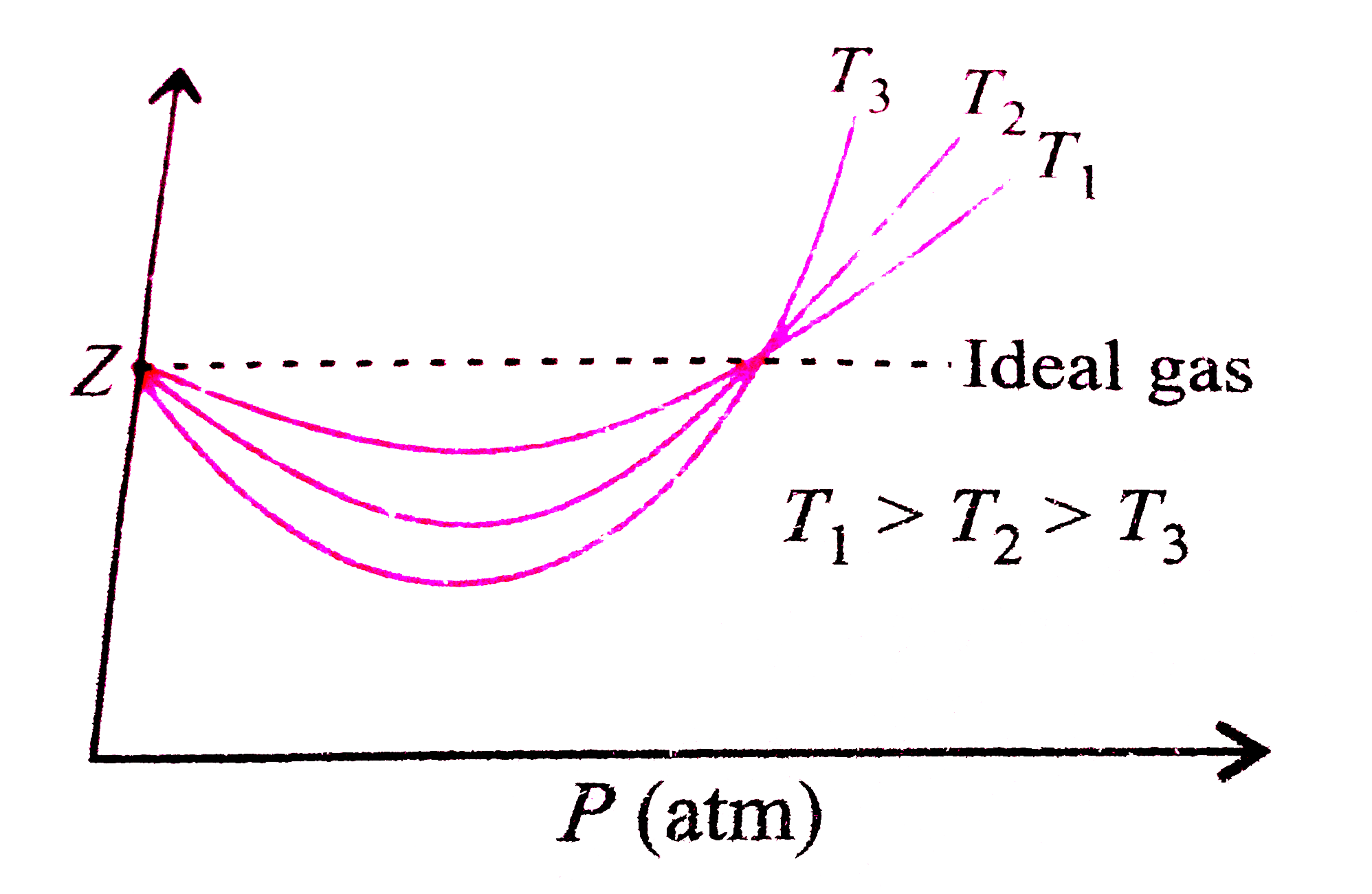
Boyle's temperature or Boyle point is the temperature at which a real gas starts behaving like an ideal gas over a particular range of pressure. A graph is plotted between the compressibility

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Chemistry!!! Not Mystery : Do Real Gases Behave Ideally?
At intermediate pressures , most gases show Z lt 1.

Ch2, Lesson E, Page 9 - Generalized Compressibility Chart

Description of real gases: Compression factor

Non-Ideal Gas Behavior Chemistry: Atoms First