
OneClass: For a real gas, the compressibility factor, Z, is
5 (681) In stock

5 (681) In stock

Compressibility factor (z): real gases deviate from ideal behav-Turito
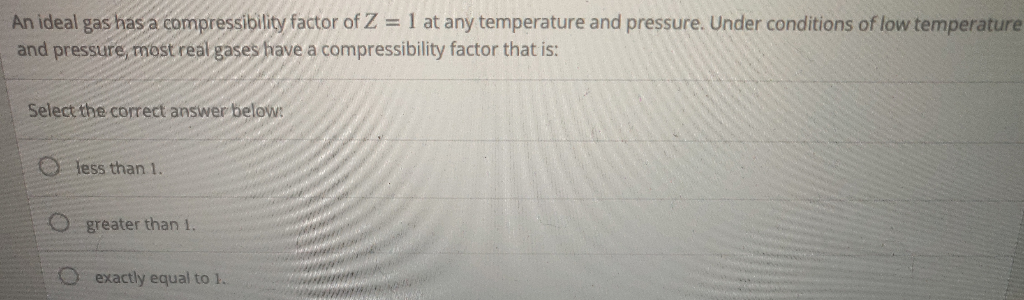
Solved An ideal gas has a compressibility factor of Z = 1 at

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
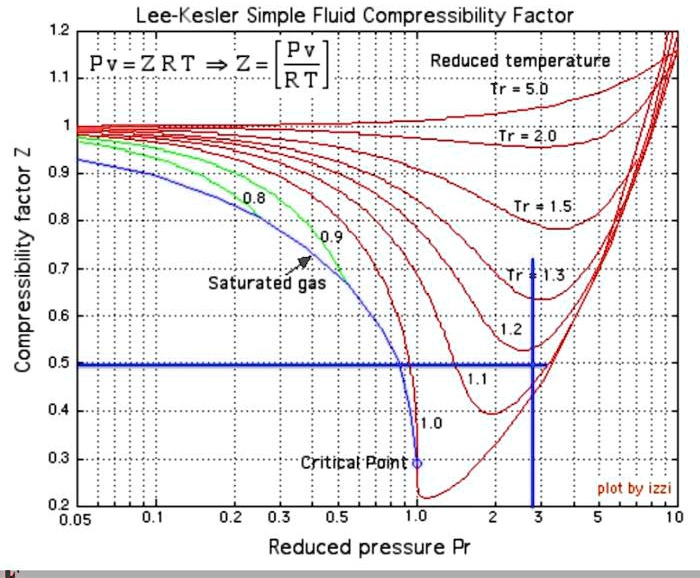
SOLVED: Lee-Kesler Simple Fluid Compressibility Factor 1.2: Pv RT Pv=ZRTZ 1.1 Reduced temperature 5.0 = 2.0 N 0.9 0.8 Compressibility factor 0.7 0.6 0.5 Tr = 0.8 0.9 Saturated gas 3 . N

States of Matter Class 11 Notes CBSE Chemistry Chapter 5 [PDF]

OneClass: Estimate the specific volume of helium at -254 degree C and 287 kPa by: (a) Ideal Gas Law (
PDF) Analysis of approximations of the gas compressibility factor derived from genetic algorithms

OneClass: 2. Fugacity for a van-der-Waals gas Let's get a feel for how much fugacity deviates from pr

For a gas at a given temperature, the compression factor is described by the empirical equa - OneClass

Compressibility Factor Z // Thermodynamics - Class 85
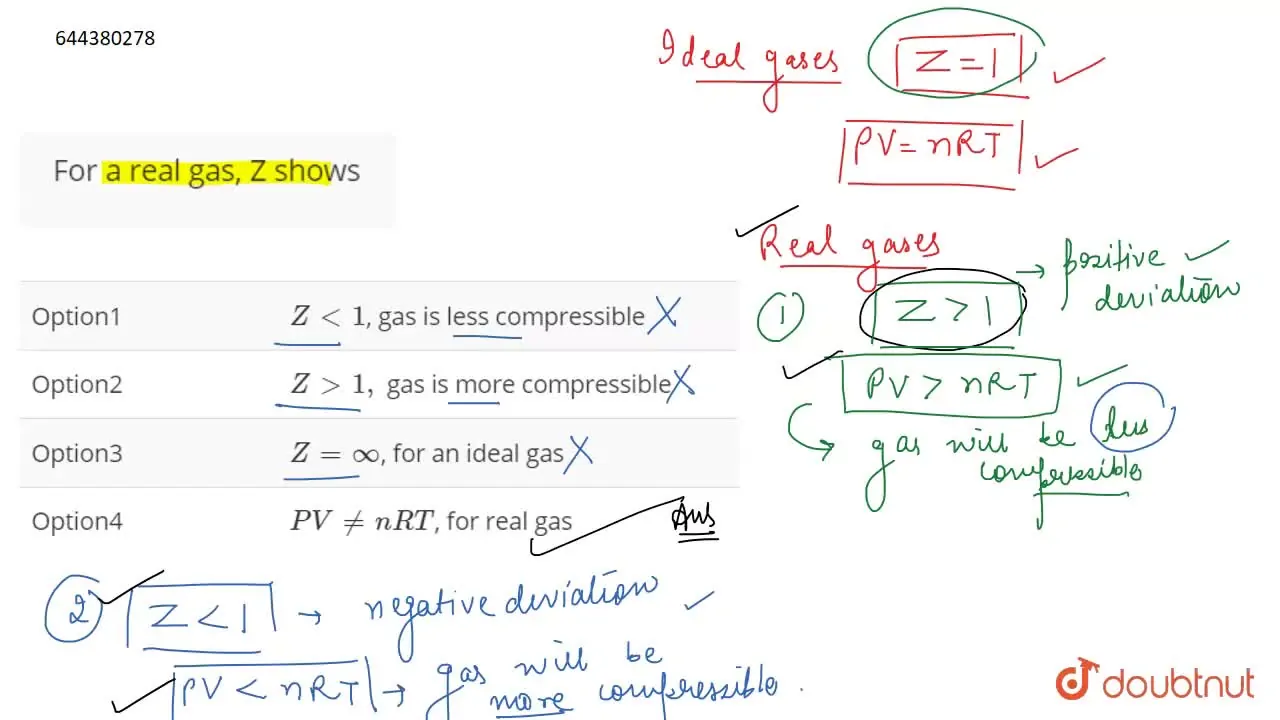
For a real gas, Z shows

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as