
kinetic theory - Why doesn't Helium behave as an ideal gas
4.7 (783) In stock

4.7 (783) In stock
I am a bit confused (might be due to some conceptual misunderstanding) as to why doesn't Helium behave as an ideal gas (it shows a deviation from the $pV$ vs $p$ graph)? (Given the fact that it is
Negative deviation in PV vs P graph
Conflicting definition of degree of freedom in Kinetic Theory of Gases
In kinetic theory, we assume that the number of molecules in a gas

The Ideal Gas Law - Video Tutorials & Practice Problems

Ideal Gas Law, Examples & Problems - Lesson

Convection, Venus, Thought Experiments and Tall Rooms Full of Gas

The Kinetic Theory of Matter - ppt video online download
Is kinetic theory applicable to ideal gas only? If yes, why is it so? - Quora

Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature

PPT - Gases – Kinetic Theory revisited (assumptions for “ Ideal” Gases) PowerPoint Presentation - ID:4342875
TIL When helium is cooled to almost absolute zero, the lowest temperature possible, it becomes a liquid with surprising properties. It can flow against gravity and will start running up and over
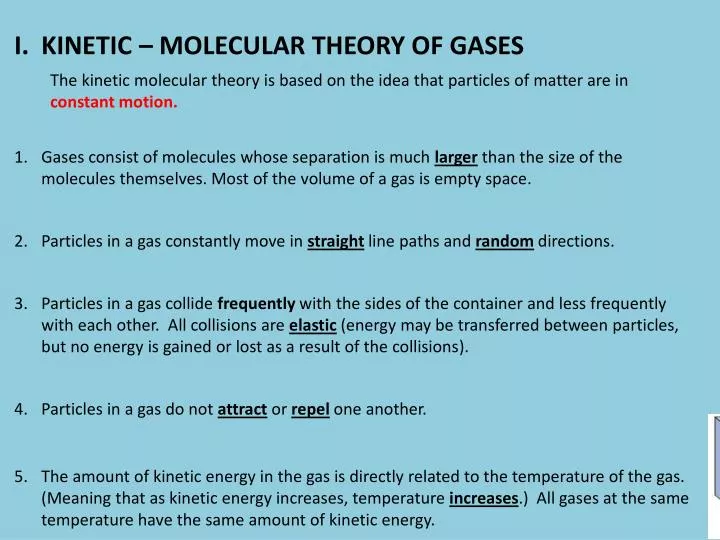
PPT - KINETIC – MOLECULAR THEORY OF GASES PowerPoint Presentation, free download - ID:4176699

Real Gas vs Ideal Gas