
Compressibility factor (Z) for a van der Waals real gas at critical point is
4.9 (782) In stock

4.9 (782) In stock
Share your videos with friends, family and the world

Bengali] What will the value of compressibility factor (Z) be for a g
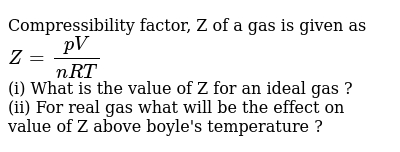
Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What

The Compression Factor, Z, and Real Gases - What you NEED to Know!
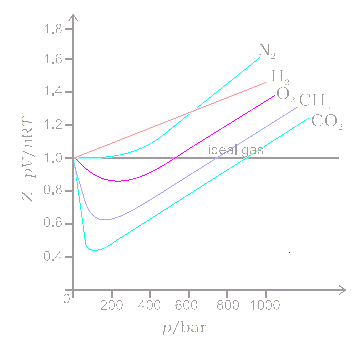
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is

For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application
Van der waals equation: Derivation, Explanation
Answer in Molecular Physics Thermodynamics for Neilmar #278440

Maxwell's speed distribution curve
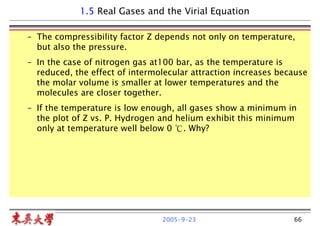
Real Gases and the Virial Equation

Telugu] Under critical states for one mole of a gas, compressibility
Van der waals equation: Derivation, Explanation
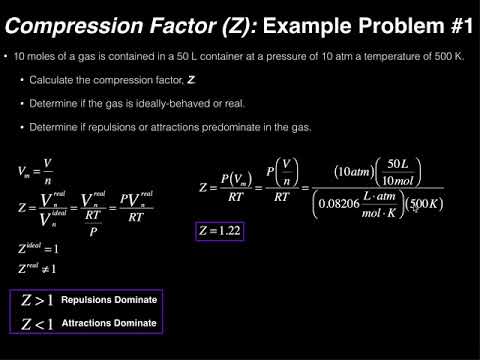
Physical Chemistry The Compression Factor (Z) [w/1 example]