
UNUB At Boyle temperature, the value of compressi factor Z has a
4.5 (698) In stock

4.5 (698) In stock
Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

What is compressibility factor? What is its value for ideal gas
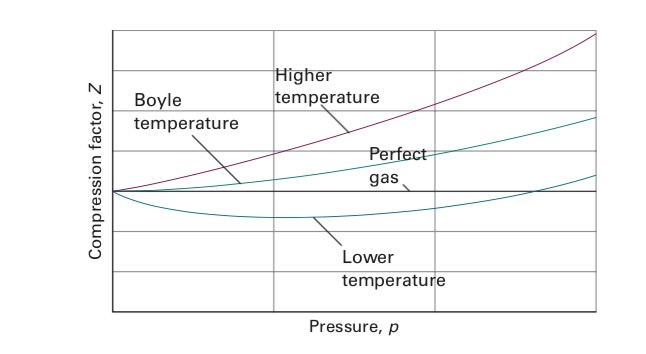
Solved I have a question about Boyle Temperature. I

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
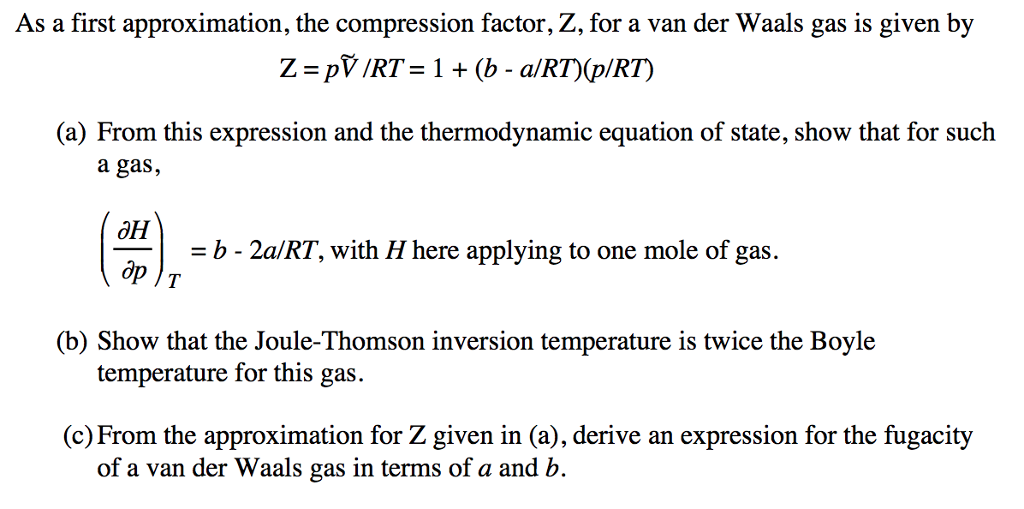
As a first approximation, the compression factor, Z

Deviation From Ideal Gas Behavior - Study Material for IIT JEE

gas laws - Compressible Factor - Chemistry Stack Exchange

PDF) Effect of Temperature and Z-Factor on Casing Designusing Kick

Course Outline: Particulate Nature of Matter, PDF, Gases

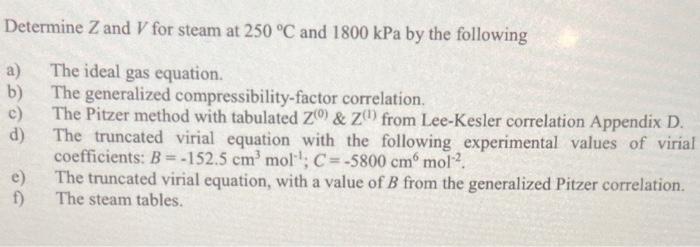
Solved Determine Z and V for steam at 250∘C and 1800kPa by

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1

PDF) Effect of Temperature and Z-Factor on Casing Designusing Kick
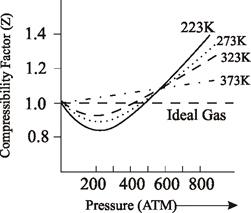
Chemistry Desk: Effect of Temperature

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is